January 03, 2022
Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine to:
- Expand the use of a single booster dose to include use in individuals12 through 15 years of age.
- Shorten the time between the completion of primary vaccination ofthe Pfizer-BioNTech COVID-19 Vaccine and a booster dose to at least five months.
- Allow for a third primary series dose for certain immunocompromised children 5 through 11 years of age.
“Throughout the pandemic, as the virus that causes COVID-19 has continuously evolved, the need for the FDA to quickly adapt has meant using the best available science to make informed decisions with the health and safety of the American public in mind,” said Acting FDA Commissioner Janet Woodcock, M.D.“With the current wave of the omicron variant, it’s critical that we continue to take effective, life-saving preventative measures such as primary vaccination and boosters, mask wearing andsocial distancing in order to effectively fight COVID-19.”
What you need to know:
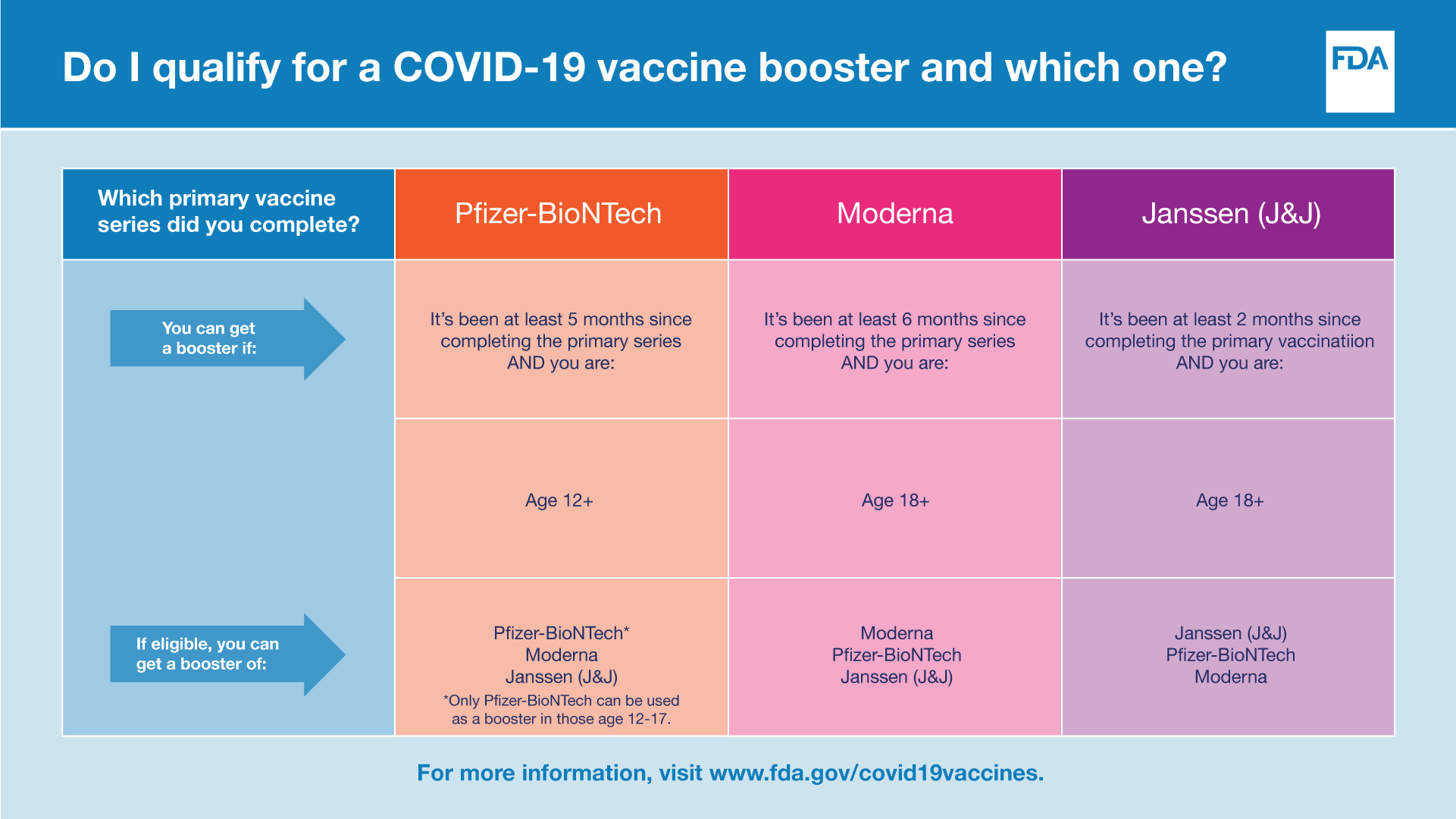
Boosters are now authorized for people 12 years of age and older
Today’s action expands the use of a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine to include its use in individuals as young as12 years of age.
The agency has determined that the protective health benefits of asingle booster dose of the Pfizer-BioNTech COVID-19 Vaccine to provide continued protection against COVID-19 and the associated serious consequences that can occur including hospitalization anddeath, outweigh the potential risks in individuals 12 through 15 yearsof age.
The FDA reviewed real-world data from Israel, including safety data from more than 6,300 individuals 12 through 15 years of age who received a booster dose of the vaccine at least 5 months following completion of the primary two-dose vaccination series.
These additional data enabled the FDA to reassess the benefits and risks of the use of a booster in the younger adolescent population in the setting of the current surge in COVID-19 cases. The data shows there are no new safety concerns following a booster in this population. There were no new cases of myocarditis orpericarditis reported to date in these individuals.
Booster interval updated to five months for people 12 years ofage and older
The FDA is also authorizing the use of a single booster dose five months after completion of the primary vaccination series of the Pfizer-BioNTech COVID-19 Vaccine.
Since Pfizer initially submitted safety and effectiveness data on asingle booster dose following primary vaccination, additional real-world data have become available on the increasing number of casesof COVID- 19 with the omicron variant in the U.S.
No new safety concerns have emerged from a population of over 4.1million individuals 16 years of age and older in Israel who received abooster dose at least five months following completion of the primaryvaccination series.
Additionally, peer-reviewed data from multiple laboratories indicate that a booster dose of the Pfizer- BioNTech COVID-19 Vaccine greatlyimproves an individual’s antibody response to be able to counter theomicron variant. Authorizing booster vaccination to take place at five months rather than six months may therefore provide betterprotection sooner for individuals against the highly transmissible omicron variant. Given the demonstrated safety and effectiveness of abooster dose when administered five months after the primary vaccination series, and the fact that a booster dose may help provide better protection against the rapidly spreading omicron variant, theFDA has determined that the known and potential benefits of administering a booster to individuals ages 12 and older at least fivemonths following completion of the primary vaccination series ,outweighs the known and potential risks.
While today’s action applies to the Pfizer-BioNTech COVID-19Vaccine, the FDA continues to review data concerning all available vaccines and will provide additional updates as appropriate.
A third primary series dose for certain immunocompromised children ages 5 through 11
Children 5 through 11 years of age who have undergone solid organ transplantation, or who have been diagnosed with conditions that are considered to have an equivalent level of immunocompromise, may not respond adequately to the two-dose primary vaccination series. Thus, a third primary series dose has now been authorized for this group. This will now allow these children to receive the maximum potential benefit from vaccination.
The FDA previously authorized a third primary series dose for use aspart of the primary immunization series in individuals 12 years and older. The potential effectiveness of an additional dose in children 5through 11 years of age was extrapolated from data in adults.
The agency used prior analyses conducted as part of theauthorization process for healthy children to inform safety in thispopulation and determined that the potential benefits of theadministration of a third primary series dose at least 28 daysfollowing the second dose of the two-dose regimen, outweighed thepotential and known risks of the vaccine. To date, the FDA and CDC have seen no new safety signals in this age group.
Children 5 through 11 years of age who are fully vaccinated and are not immunocompromised do not need a third dose at this time, but the FDA will continue to review information and communicate with the public if data emerges suggesting booster doses are needed for this pediatric population.
“Based on the FDA’s assessment of currently available data, a booster dose of the currently authorized vaccines may help provide better protection against both the delta and omicron variants. In particular, the omicron variant appears to be more resistant to the antibody levels produced in response to theprimary series doses from the current vaccines,” said PeterMarks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “With this in mind, the FDA has extended the range of individuals eligible to receive a booster, shortened the length of time between the completion of the Pfizer primary series for individuals to receive a booster, and is authorizing a third protective vaccine dose for some of our youngest and most vulnerable individuals.”
The fact sheets for recipients and caregivers and for healthcare providerscontain information about the potential side effects, as well as the risks of myocarditis and pericarditis. The FDA and the U.S. Centers for DiseaseControl and Prevention have several systems (https://www.fda.gov/vaccines-blood-biologics/safety- availability-biologics/covid-19-vaccine-safety-surveillance) in place to continually monitor COVID-19 vaccine safety and allow for the rapid detection and investigation of potential safety concerns.
The most commonly reported side effects by individuals who received abooster dose or an additional dose as part of a primary series were pain, redness and swelling at the injection site, as well as fatigue, headache, muscle or joint pain and chills. Of note, swollen lymph nodes in the underarm were observed more frequently following the booster dose than after the second dose of a two-dose primary series.
The FDA will publicly post documents regarding the agency’s decision onits website following authorization.
The amendment to the EUA was granted to Pfizer Inc.
Related Information
Pfizer-BioNTech COVID-19 Vaccine(https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid- 19/comirnaty-and-pfizer-biontech-covid-19-vaccine)
COVID-19 Vaccines (https://www.fda.gov/emergency- preparedness-and-response/coronavirus-disease- 2019-covid-19/covid-19-vaccines)
Emergency Use Authorization for Vaccines Explained(https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization- vaccines-explained)
Consumer:
# 888-INFO-FDA