New Alzheimer’s Drug Shows Promise
New reports show that an experimental Alzheimer’s medication successfully slowed declines in the ability to perform tasks and think clearly in more than a third of the individuals who participated in a large clinical trial. Positive reports as a result of the trial have prompted Eli Lilly, the drug’s manufacturer, to file for FDA approval by the end of June. The new drug will be recommended for use in individuals who have early symptoms of Alzheimer’s disease.
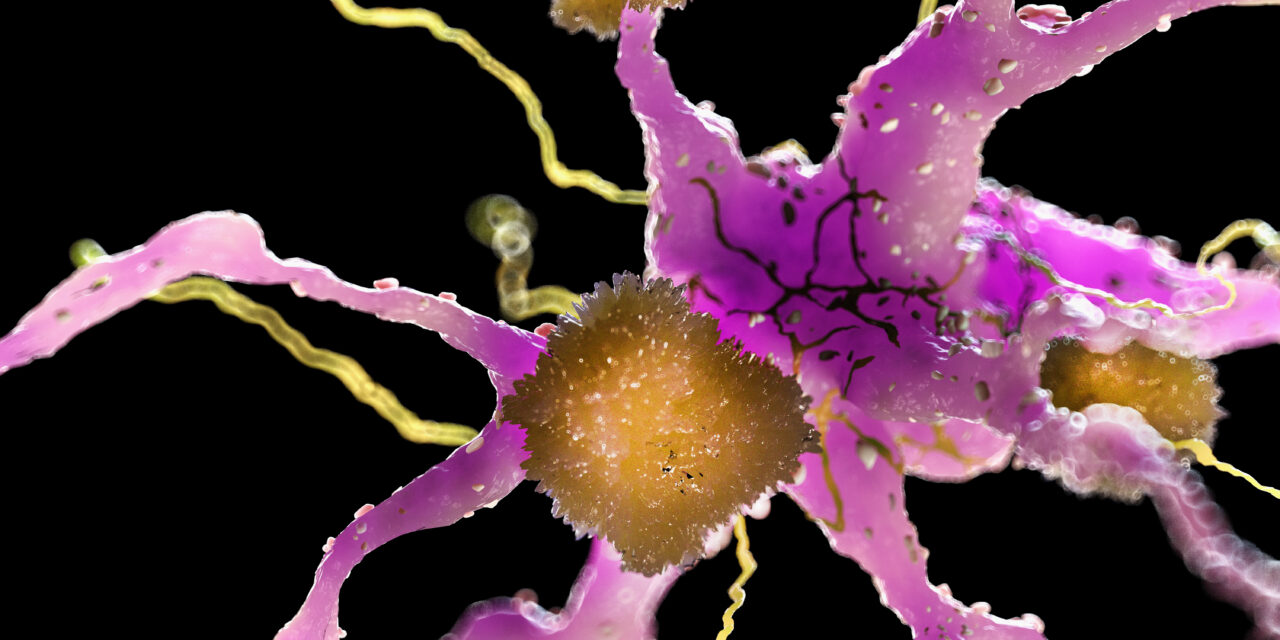
The medicine, known as donanemab, removes amyloid plaque buildups in the brain that are a hallmark sign of Alzheimer’s disease. There were three deaths among the more than 1,700 trial participants taking the drug over a period of 18 months. Researchers say their deaths were due to brain swelling or microhemorrhages, known as amyloid-related imaging abnormalities or ARIA.
Dr. Daniel Skovronsky, Eli Lilly’s Chief Scientific and Medical Officer, points out that, “For every medicine, for every disease, there are potential risks and potential benefits.” Skovronsky notes that almost half of the participants taking the drug, 47%, showed no decline on a key measure of cognition over the course of a year, compared with 29% of individuals who were taking a placebo. He says, “That’s the kind of efficacy that’s never been seen before in Alzheimer’s disease.”
Eli Lilly notes that Alzheimer’s affects more than 6 million Americans, with an estimated 1.7 million to 2 million people over 65 experiencing early stages of the disease. It is encouraging that donanemab shows promise.
Learn more about the results of the donanemab clinical trial from a recent statement issued by the Alzheimer’s Association at https://www.alz.org/news/2023/association-statement-donanemab.